Abstract
Background: Dehydrated hereditary stomatocytosis (DHSt), also known as hereditary xerocytosis, is a rare congenital hemolytic anemia with an autosomal dominant inheritance. It is often misdiagnosed for other hemolytic conditions, such as hereditary spherocytosis. Herein, we present the case of a young female presenting with hemolytic anemia, who was found to have a mutation in PIEZO1 gene and was subsequently diagnosed with DHSt.
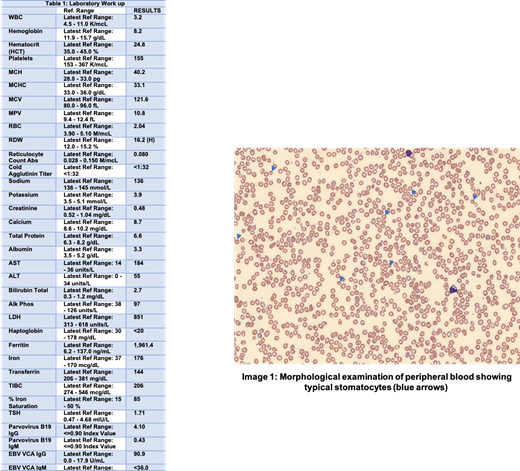
Case Presentation: A 39-year-old woman of Asian origin presented to the hematology clinic for evaluation of anemia diagnosed on blood work performed by primary care physician for symptoms of fatigue. She was adopted and had no information about her family history. A complete blood count revealed: hemoglobin 8.2 g/dL, mean corpuscular volume (MCV) 121.6 fL, absolute retic counts 0.08 M/mcL, lactate dehydrogenase 851 units/L and a negative coombs test. Iron profile revealed iron saturation 85% and ferritin 1961.4 ng/mL (see table 1 for laboratory work up). The peripheral blood smear showed anisopoikilocytosis, macrocytes, spherocytes and several stomatocytes along with polychromatophils. An ultrasound of the abdomen was subsequently performed, and which revealed hepatomegaly and biliary stones. Enzyme assay for glucose-6-phosphate dehydrogenase and flow cytometry for paroxysmal nocturnal hemoglobinuria were also sent and were negative. Red blood cells osmotic fragility was decreased. The bone marrow biopsy showed full spectrum trilineage hematopoiesis with no mutations on molecular testing. Based on the blood smear and clinical presentation, a diagnosis of DHSt was suspected. Genetic testing was performed and which revealed Sc.2842C>T; p.Arg948Cys mutation in the PIEZO1 gene by massively parallel sequencing and confirmation by Sanger sequencing. This confirmed the diagnosis of DHSt. Patient was started on high dose folic acid with improvement in her hemoglobin in one month. She did not require any blood transfusions. MRI liver T2* scan measured quantitative liver iron of 31 mM/g, which was at the high normal range.
Discussion: DHSt is caused by gain of function mutation in PIEZO1 gene or KCCN4 gene which encode the transmembrane cation ion channel and Gardo's channel respectively on red blood cell membrane. This results in delayed inactivation of the channel. The disease presents as a spectrum from asymptomatic anemia to massive hemolysis, and many patients present later in life. Patients may manifest clinical signs of jaundice, pallor, fatigue, splenomegaly, gallstones and iron overload. Labs are typically significant for elevation in mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW) and MCV, with classic slit cells red blood cells seen on peripheral blood smear (see image 1). PIEZO1 is expressed early in erythroid progenitor cells and may delay erythroid differentiation and reticulocyte maturation, which may be the cause of low reticulocyte count such as in our patient. While treatment is supportive with blood transfusions, only a minority of DHSt patients ever require regular transfusions. Interestingly, hyperferritinemia, high transferrin saturation or clinical iron overload are quite frequent in DHSt and iron chelation is recommended. Splenectomy is contraindicated due to increased risk of thrombosis.
Conclusions: DHSt as a rare inherited hemolytic anemia and its diagnosis warrants maintaining a high index of clinical suspicion based on supportive laboratory findings. Diagnosis involves thorough testing earlier in the disease as patients may be asymptomatic until adulthood. Delaying the diagnosis may lead to severe iron overload and consequent organ damage.
No relevant conflicts of interest to declare.